Welcome to
spm² – safety projects & more GmbH
spm² is a Germany based specialized (pharmaco-)vigilance CRO located in Hirschberg a.d. Bergstraße about 13 km north of Heidelberg
spm² was founded in 2005 and is managed by Petra Sujatta, Dr. Martin Sujatta and Dr. Diana Witticke.
spm² is a sister company to spmd – safety strategies for health Inc., located in Topsfield, MA in the north of Boston, MA, USA which is well-established since 2016 and specializes in (pharmaco-)vigilance services for the US.
spm² offers the full range of (pharmaco-)vigilance services for clients worldwide and for the entire product lifecycle from discovery to post-marketing.
spm²’s focus is to support clinical trial programs, regulatory applications, as well as post-marketing surveillance in Europe and the US.

Dr. med. Dipl. biol. Martin Sujatta
Senior Partner
Physician Consultant
Executive Management

Dr. Diana Witticke
Senior Partner
Senior Vice President
General Manager

Dipl. Inform. med. Petra Sujatta
Senior Partner
IT Responsible Person
General Manager










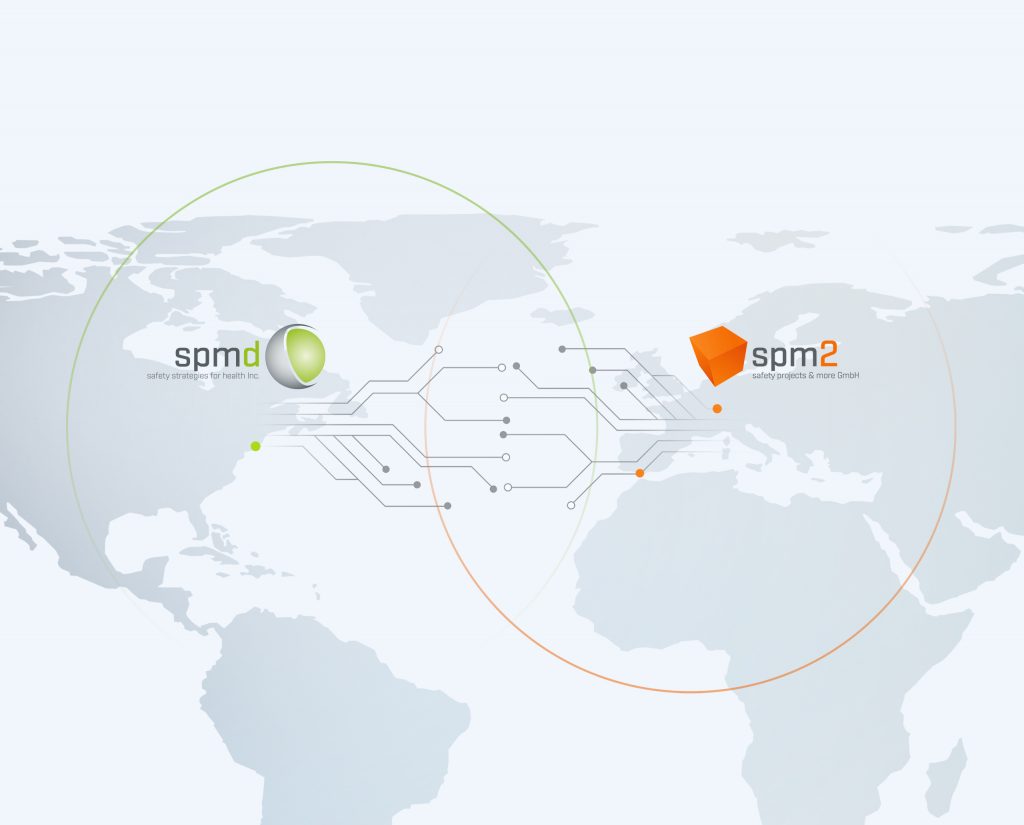
GLOBAL MARKETS
EU/Germany based spm² and USA based spmd have entered into an exclusive cooperation agreement
GLOBAL MARKETS
EU/Germany based spm² and USA based spmd have entered into an exclusive cooperation agreement
STATEMENT

We are committed to provide state of the art safety solutions tailored to our clients’ needs for the protection and welfare of patients and healthcare providers.
It is our aim to establish long-term business relationships and to provide our clients with value-adding solutions.
IN OUR EMPLOYEES
In an environment of greater regulatory enforcement and increased accountability, our dedicated safety teams assist our clients in ensuring patient safety, full compliance with regulatory and legal requirements, and driving project success.
Our team is comprised of highly-skilled healthcare professionals, scientists, and IT specialists with long-standing experience in basic research, hospital care, and clinical development – in the USA and European regulatory environment.
IN OUR DAILY WORK
We are focused on areas where we can achieve a competitive edge through the excellent quality of our services which are standardized for optimal efficiency.
It is our aim to establish long-term business relationships and to provide our clients with tailor-made solutions.
INSPIRED BY SAFETY!
Welcome at spm² – safety projects & more GmbH
Explore the world of (pharmaco-)vigilance and how we can support you…

spm² – safety projects & more GmbH
Goldbeckstraße 5,
69493 Hirschberg an der Bergstraße
Phone: +49 (0) 6201 – 846 40-0
Email: info@spm2-safety.com